Controls prevent errors
Design documentation seems daunting when we consider the way a government defines its regulations. However, our firm defines clear processes that simplify the organization and methods to meet requirements, while improving the quality and safety of products.
Design Documents
- Document user, performance, and regulatory needs
- Define testing and document the results
- Define labeling for safety and user experience
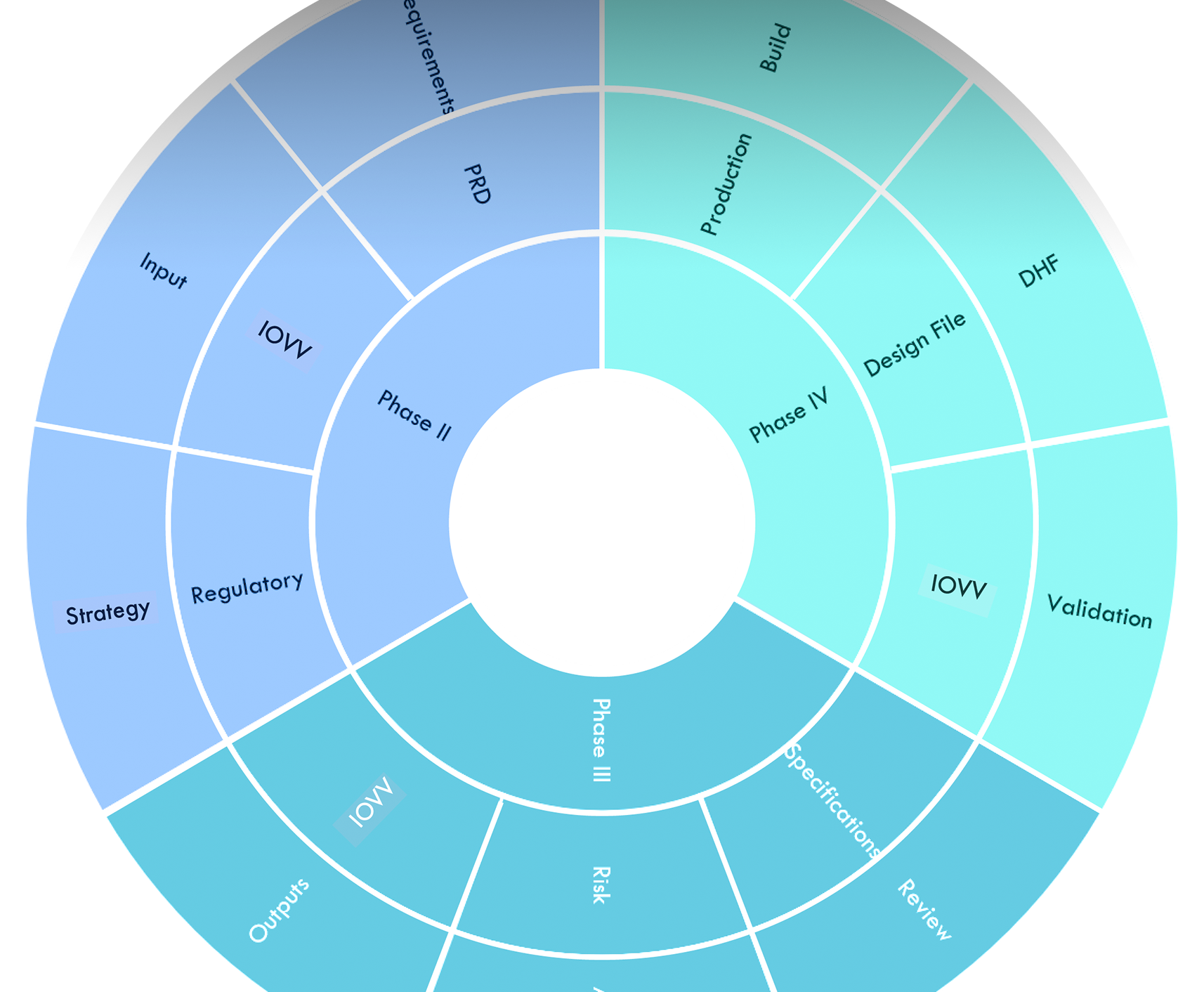
Regulatory Documents
A 510k filing can be comprised of thousands of pages. Several design processes may only require a few dozen pages. The key is a process like ours that can accommodate all levels of client needs.
What Documents Do I Need?
And when do I need design controls?
Design Control Process
Safety and risk documents are the minimal considerations a device manufacturer should consider when developing a device.
Although many agencies allow self-regulating on low-level devices, the accuracy of a manufacturer's self-regulated records can be questioned during audits or lawsuits.
Higher-risk devices require a more stringent and controlled design process conforming to 21 CFR 820.30 and EU MedDev requirements.
This list represents the bulk share of the documents needed to produce a sufficient design file. Each item requires team interaction and a high level of detailed understanding of device design and regulations associated with your product code.
Payback
In 2017, baby Luke was born to the family of a Zewski team member, and our employees were able to see the products they designed, firsthand and personal.
