Having had an opportunity to work on research and development engineering teams for products such as medication infusion sets, tissue heart valves, and neonatal medical equipment, I’ve been able to observe the medical device design process from multiple angles. The design process starts with a medical need, such as a way to deliver medication subcutaneously to a patient safely, painlessly, and reliably.
Brainstorming
Brainstorming occurs next. “Blue Sky” or brand new ideas can often emerge in direct response to the medical need or during a brainstorming session, but many times market research is needed regarding methods and devices already available that meet this need; if there is room for improvement to existing methods, “derivative” ideas which improve upon an existing method or device can emerge during the brainstorming sessions.
A common or traditional approach to the brainstorming phase is for idea generation to come solely from R&D engineers and industrial designers, but in my experience, it is ideal if the brainstorming phase includes people from various backgrounds and varying levels of experience, not solely technical, and not necessarily with a great number of years of experience. Sometimes the best ideas come directly from customers or from those experienced in the manufacturing or testing of devices. Feedback from clinicians, marketing research, and customer support calls is invaluable in addressing design changes needed for existing products. In addition, fresh ideas and perspectives can be offered by more junior team members. A beautiful free-flow of ideas can occur during this phase when all feel respected and heard, and when one idea builds off of another even if in unpredictable ways. The absolute best ideas are generated in this type of environment, and it bodes well for a smooth development process if all key team members feel they have had buy-in. Sometimes this is just not possible, and ultimately the technical feasibility needs to be established by R&D engineers responsible for the project, but the brainstorming process is precisely for looking at the widest possible range of ideas before narrowing down to the most feasible.
Development
Once the brainstorming phase has yielded some promising ideas, a search of existing patents is important to make sure ideas are not pursued that are already existing and legally protected. Sometimes management teams believe it is valuable to conduct this research before the brainstorming session, and this certainly has its merits, but it can limit idea generation or steer it down already well-trodden paths. A balance should be struck between spending too much time “reinventing the wheel” and getting stuck in existing thought processes. One way to ensure this is to set time limits on how long “Blue-Sky” brainstorming can take place.
Preliminary product specifications are generated at this point as well – what will the new device need to do, and how will it need to do it? Numbers should be inserted wherever possible, but the team should not get stuck if all numbers aren’t available – bench-testing prototypes often helps fill in these gaps. The design process is iterative and cyclical, not directly linear.
Design sketches and CAD models are produced next. This serves two purposes – to better communicate designs to the project team, and to more fully flesh out ideas. Often it is not until a design is sketched or modeled that previously unexamined details emerge. An initial prototype can then be built at this point, using a 3D printer, machining instead of molding if the design is to be made of plastic, or by assembling modified, commercially-available, similar-enough parts. This further fleshes out details not detected during the sketching or CAD modeling step. The modeling/initial prototyping process is cyclical – information gleaned from the initial prototyping step can be used to update the design sketch and CAD model. This cycle continues as long as necessary for a satisfying approximation of the design to be achieved.
In parallel with the sketching/modeling/initial prototyping process is research into manufacturing methods, feasible materials, possible vendors, testing methods, and packaging and sterilization needs. This research “builds a case” for design feasibility. A beautiful design that can’t be built, manufactured within budget, manufactured using materials that are biocompatible, can’t be tested to specifications, or that can’t be reasonably sterilized or packaged is not a feasible design. The results of research into each of these areas in turn is used to update the design along the way – again this is a cyclical, iterative process rather than a linear one. The design process is about optimization – this is the “development” portion of “R&D” – developing one feasible idea into a product that meets as many of the stated goals and requirements as possible.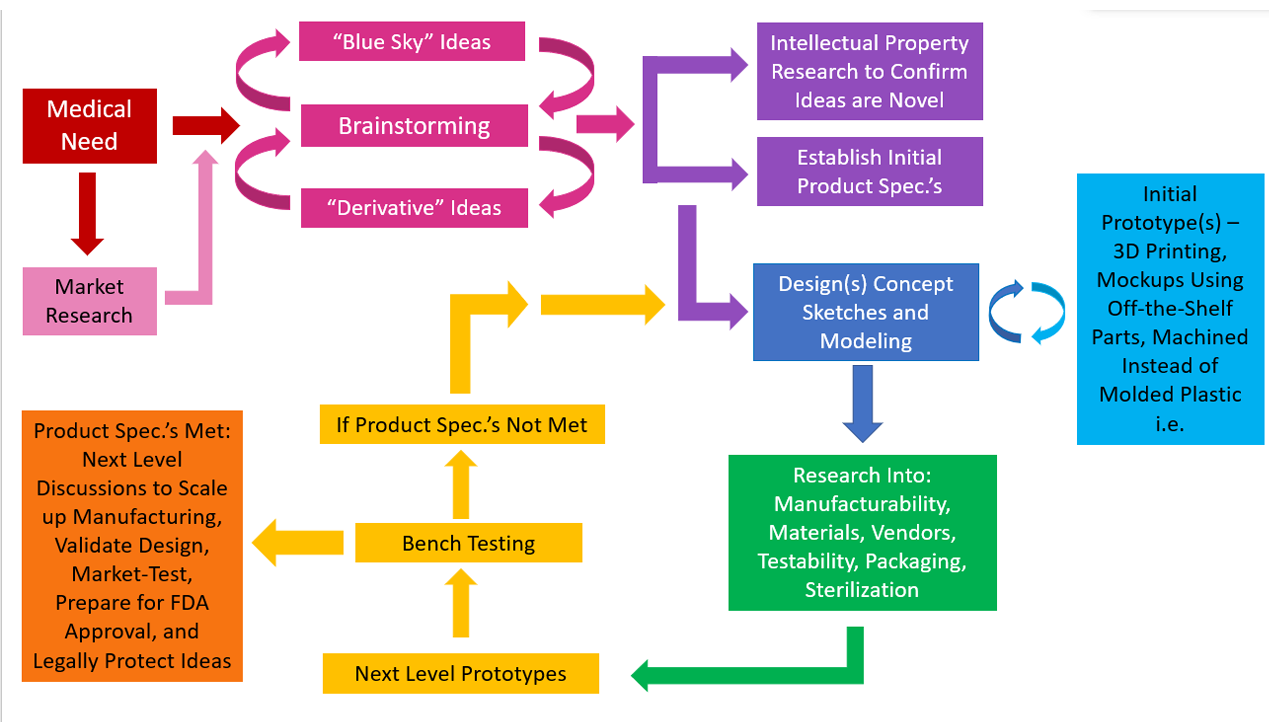
Verification and Validation
The next stage is to work with vendors to make a large number of next-level prototype parts than can be assembled, if necessary, by a third-party vendor or in-house. These prototype parts will need to be tested against the product specification, by a third-party vendor or in-house. If the specifications are not met, both the design and manufacturing need to be revisited, or the product specifications adjusted to meet the reality of the design. In this case the entire team would need to be consulted to ensure the new specifications were acceptable. It is important to order enough parts for testing that proper statistics can be applied. The standard deviations resulting from the tests can’t be known until testing occurs, so a large sample size must be planned for when ordering parts.
Design Transfer
If the product specifications are met, meetings about bringing the design to market can then be held between the R&D team and Manufacturing, Quality Assurance, Regulatory Affairs, Marketing, and Legal. Meetings with Manufacturing and Quality will determine how the design is to be manufactured at a larger scale, and to what technical standards it will be held. Regulatory will be responsible for ensuring the design obtains FDA approval. Marketing will communicate with the customer base and truly be responsible for bringing the design to market. Legal will aid in submitting patent applications in order to protect all feasible design iterations.
This high-level summary of the medical device design process doesn’t discuss the budgeting of time or money and doesn’t detail all parties involved at every step of the process, but it certainly includes the major phases and discusses how they relate. Medical device design can be fun and exciting in that it presents many unique challenges and the end result can have a hugely positive impact on a large number of people.

