Maximizing Global Health by considering Open-Source Options
Introduction
Research institutions have great programs that encourage scientists, clinicians, and engineers to solve complex problems and think out of the box. Many institutions run several hundred research projects a year. However, because institutions typically produce only early-stage research and limited proof-of-concept (POC) designs, only large medtech manufacturers can afford to invest in the expensive continued design of the product for production and fully de-risk it through testing. These large firms are very selective, only bringing a small percentage of POC designs to market, leaving both clinical research and educational institutions with many potentially groundbreaking products with no path forward. Many of these ideas are great candidates for the open-source global health arena and could improve global health, but historically open-source ideas seldom gain the attention of industry. Increasing the number of designs that eventually improve the health of patients requires understanding the amount of effort that goes into the total design development process of a medical device and who pays for this development.
Understanding the Stages of Development
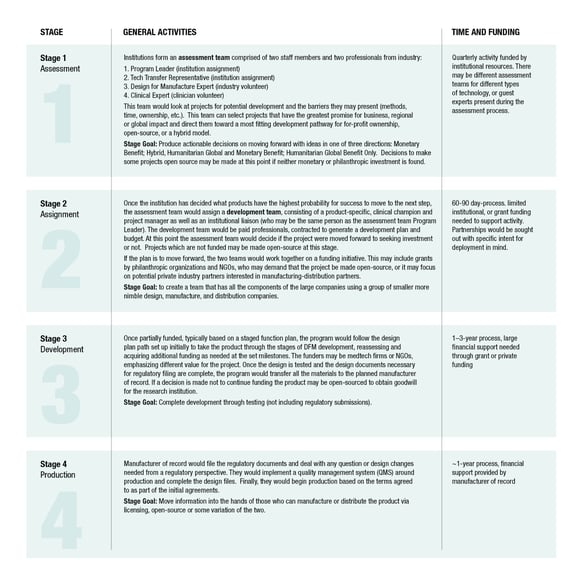
To understand the best way to transition technology, its import to differentiate POC research conducted at institutions vs work from a production-ready development standpoint, or design for manufacture (DFM). A POC is an essential step from a theoretical concept to a practical device, and quickly tests if the concept makes sense. A POC may be useful to motivate more investors and institutions involved in the development process. It is not a commercially viable production-ready design because it is not optimized for cost nor has it been developed with the rigor needed for regulatory approval. This additional design work is generally less interesting to research institutions, so the great preponderance of POCs which are not selected by large firms for further development are simply never developed further, and, if they are never released under an open-source license, simply lost to the world.
The full development process from POC to production is a multi-stage journey called design-for-manufacturing (DFM). The DFM process, regarding medical technology, is part of a larger regulatory approval process required by governmental regulations. It is a meticulous process encompassing multiple steps from decision-making to problem-solving. Large-scale manufacturers and experienced engineers already have a defined DFM process that they follow, allowing them to efficiently develop a product, minimizing cost, time, and effort. Although the DFM process at each company may vary slightly, the essentials remain the same. A prototype (no matter what stage it is in – alpha, beta, production) represents the final product more accurately than a POC and it is usually the result of rethinking the POC in that structured process making the design comparatively easier to translate into a high-volume production-ready design. At an early stage in the DFM process, experienced engineers consider:
- ideas that can help save costs and time in production,
- ways to reduce the number of components or completely redesigning parts to ease the manufacturing process, and
- study other industries to find solutions and components that perform the required work with less lead time and costs.
As part of the regulatory aspect of the DFM process, engineers will study international (ISO) standards, and develop test plans to evaluate the product's compliance with the regulatory and safety requirements. Because part of the controlled DFM process in the medical industry includes developing manufacturing processes during the development of the device, experienced engineers who understands best practices for manufacturing will be needed to design the product. Additionally, these engineers will assist the manufacturer in developing processes to regulate the manufacturing and quality of the product, creating a seamless transition to production. These activities will generally not be of interest to institutional researchers.
Philanthropic Impact Full Open-Source Model
We do not imagine that open-source designs which have not gone through a rigorous regulatory process, including design-for-manufacture, will have a practical impact on health except under exceptionally dire circumstances. For example, in the USA the FDA did create a relaxed process of Emergency Use Authorization for devices, especially ventilators, during the early COVID-19 pandemic. However, this was still a regulated process. Therefore, if a research institution makes a POC open-source, it may obtain goodwill and reputation, but most such designs will not be brought to market unless a humanitarian organization subsidizes the design-for-manufacture cost. However, a research institution obtains more benefit in renown and reputation from making a POC open-source than from freezing development in a closed-source state which will provide them zero benefits.
Research Institutions, Industry and Funders
Coming up with a proof-of-concept for an idea can be challenging, requiring individuals with training in the sciences and creative, divergent thinking and the ability to solve complex problems quickly. Institutions have direct access to such highly skilled individuals, usually already on staff, who can work on these POCs, effectively functioning as an advanced R&D department.
Similarly, large medtech companies have in-house experts – ‘advanced R&D’ departments in which Ph.D. level educated staff, develop POCs to create ideas of tomorrow. But they also have conventional R&D, manufacturing departments, and sales and distribution channels all under one roof.
This makes large companies ideal partners for research institutions to develop their ideas and bring them to market. However, these large companies have significant restrictions, including boards to report to, large scale profit margins to consider, conflicts of interest in other business they control, etc. As a result of all these factors big medtech is highly selective with the institutional partnerships leading to the result that only a handful of potential health-improving ideas selected from the many thousand ever see the market and help patients.
However, working with clinical inventors, research institutions, manufacturers, global health experts, and philanthropic NGOs, we believe there is a pathway to patients’ health for more projects via open-source development which also benefits research institutions.
The Model
Research institutions do have the ability to align themselves with a method around assessing what ideas they have that are viable for manufacturing and the market, rather than waiting for large medtech firms as the sole means for advancement. The following model has four stages for moving ideas to production using this cooperative process for either traditionally closed-source proprietary development or open-source development for global impact, or a hybrid of the two, as shown below.
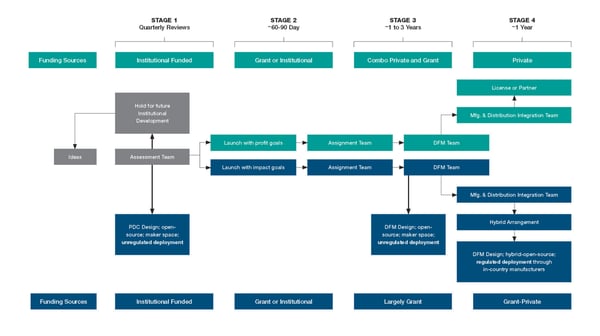 In the figure above, the upper boxes in green represent the traditional technology transfer route of attempting to obtain royalties from profits based on proprietary development. The lower boxes in blue represent our proposed alternatives of open-source development. In both cases the four stages of Assessment, Assignment, Development and Production are practically the same. However, the source of funding may differ. When an NGO or philanthropic firm de-risks a project by providing funding, one generally expects that a firm has lower risk but may have lower potential reward, either because the project had lower potential profit in the first place or because the NGO insists on expanding competition based on the technology they fund.
In the figure above, the upper boxes in green represent the traditional technology transfer route of attempting to obtain royalties from profits based on proprietary development. The lower boxes in blue represent our proposed alternatives of open-source development. In both cases the four stages of Assessment, Assignment, Development and Production are practically the same. However, the source of funding may differ. When an NGO or philanthropic firm de-risks a project by providing funding, one generally expects that a firm has lower risk but may have lower potential reward, either because the project had lower potential profit in the first place or because the NGO insists on expanding competition based on the technology they fund.
 Philanthropic Support for Medical Devices
Philanthropic Support for Medical Devices
Many small to medium device manufactures will shy away from products that they would ordinarily be glad to add to their products line, but for which the development costs pose an insurmountable risk. By working with them and de-risking the product by covering the development costs for them, NGOs and philanthropic foundations can ensure a product comes to market that would otherwise never be developed. However, charitable donors do not wish to favor one particular firm over others and may even be unable to do so and retain their charitable status. A solution is for them to pay for the design-for-manufacture of open-source devices and insist on the sharing of the resulting DFM developed with their money. The open sourcing of such a design may occur immediately, or sometime after the initial firm has begun marketing to give that firm a temporary monopoly as an incentive.
This offers an opportunity for lower-dollar-per-capita health markets such as low-and-middle income countries (LMICs) in need of low-cost manufacturing to build in-country manufacturing facilities for the same products. These devices would have to be manufactured the same way as intended during development for manufacture and receiving regulatory approval, independent of country, which is necessary for regulatory approvals. With the support of the original manufacturer incentivized by an NGO, in-country manufacturing can be set up across the globe in disadvantaged nations. This is not without challenges, but we believe that, in the long term, implementing this model successfully in a few areas will create an established pathway that will mitigate supply chain crises such as those we are currently experiencing. During future pandemics or other health crises local manufacturers can reduce supply chain issues and increase response time, while improving the local economy, all goals supported by some NGOs.
Summary
We have proposed a four-stage process for research institutions consisting of Assessment, Assignment, Development, and Production. This process is designed to maximize the benefits to a research institution from its proof-of-concept research. At each stage, there are opportunities for a device which is not considered sufficiently profitable to attract traditional investment to be supported by a philanthropic funder who emphasizes the product’s impact to global health. Similarly, at each stage, an institution may choose to open-source the design either as part of an agreement with a funder or to obtain goodwill and reputation. This proposed model benefits institutions, medtech firms, design firms, and the health of the entire globe.
- Educational and clinical institutions receive more recognition for their ability to deliver ideas that impact global health.
- Researchers, students, and clinicians get the opportunity to obtain more research value for their work and see the development process unfold as part of their postgraduate or career experience.
- Technology transfer departments can potentially recover more royalties for their departments to use for additional idea development or receive more intangible goodwill.
- Philanthropic organizations have an opportunity to invest in a greater number of projects with real-world impact including in-country development of more affordable solutions to global health.
- Small manufacturers gain access to opportunities they otherwise would not have. Independent device design professionals will gain more experience and knowledge that is often reserved for those who work at large medtech companies.

